antibody
Antibodies are proteins produced by b-cells, that specifically target antigens, during the adaptive immune response.
Tip
The structure of a b-cell receptor is identical to that of its corresponding antibody except for a small portion of the carboxy-terminus of the heavy chain Fc region. In the BCR the C-terminus is a hydrophobic amino acid sequence that anchors the molecule in the membrane, and in the antibody it is a hydrophilic sequence that allows secretion.
Structure
![[CleanShot 2023-10-11 at 23.16.52@2x.png|200]]
In any given immunoglobulin molecule the HC and LC pairs are identical therefore increasing the ability to bind simultaneously to two identical antigen on a surface, increasing the total strength of the interaction, which is called its avidity. The strength of interaction between 1 binding site and its antigen is called its affinity.
![[CleanShot 2023-10-11 at 23.43.30@2x.png|500]]
Each Ig domain is constructed from two β sheets (each made up from several β strands) folded into a β sandwich and are covalently linked by a disulphide bond, this structure is known as the immunoglobulin fold.
Tip
The constant domain folds have a 4 + 3 β sheet arrangement whereas the variable domain have a 4 + 5 β sheet arrangement at the C-terminus of the V Ig domain
The loops between the β strands in the V domain form the CDRs. When both V domains (VH and VL) come together, the six hyper-variable loops come together they form a single site at the tip of each arm of the antibody.
Antigen binding
Antibodies bind to conformational similar antigens using noncovalent forces. Antibodies generally only recognise a small portion on the surface of the target known as the epitope. In a folded protein, binding occurs over different sections of the sequence and is known as a discontinuous epitope.
Note
The interaction between antibody and its antigen can be disrupted by high salt concentrations, extreme pH, detergents and competition of other epitopes. The binding is a reversible noncovalent interaction, governed by electrostatic interactions.
Virtually any substance can be the target of an antibody response. The total number of antibody specificities available to an individual is known as the antibody repertoire, and in humans is at least 1011. The number of antibody specificities present at any one time is limited to the total number of b-cells in an individual.
Paratope (CDR) diversity
The diversity of the immunoglobulin is generated by four main processes:
- combinatorial diversity - multiple copies of each gene segment.
- junctional diversity - addition and subtraction of nucleotides at the junctions of gene segments.
- Combination of heavy & light chain V regions that pair to for the antigen-binding site. Note some VH - VL combinations are unstable.
- somatic hypermutation
Heavy chain diversity
The effector function of an antibody is defined by the heavy chain isotype.
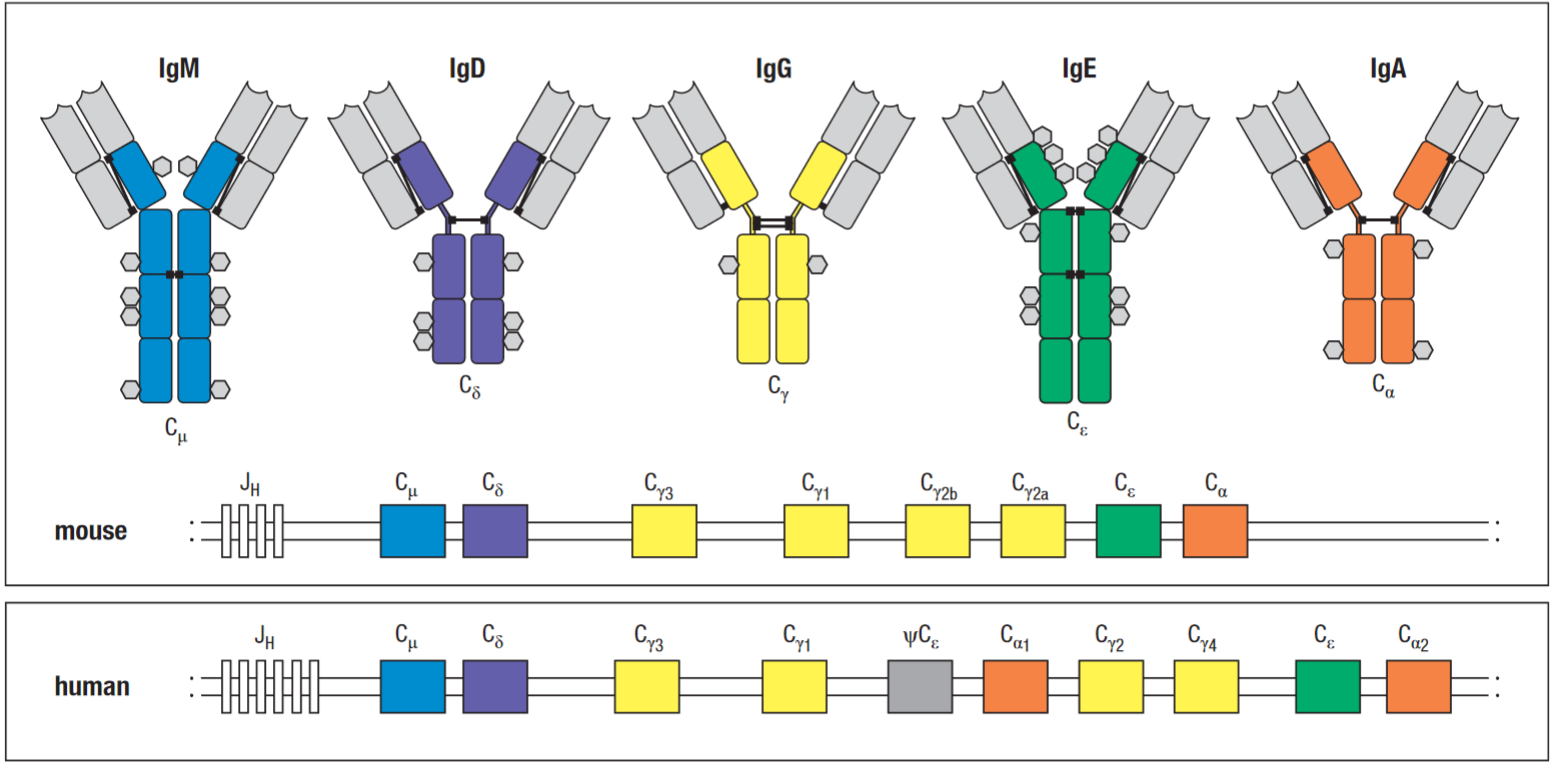
There are five classes (isotypes) of human antibodies:
- IgM antibodies - µ
- IgD antibodies - δ
- IgG antibodies - ɣ - (IgG1, 2, 3, 4 - named in decreasing order of their abundance in serum)
- IgA antibodies - α - (IgA1 & IgA2)
- IgE antibodies - ɛ
The distinct functional properties of the different classes and subclasses of antibodies are conferred by the carboxyl-terminal part of the heavy chain (see Fc domain).
The light chain C regions provide only structural attachment for V regions. Heavy chain locus encodes different C regions (CH) that are present as separate genes located downstream of the V region segments. Initially, naïve b-cells use only the first two of these, the Cμ and Cδ genes, which are expressed along with the associated assembled V-region sequence to produce transmembrane IgM and IgD on the surface of the naïve b-cell.
The same antibody gene can generate both membrane bound immunoglobulin and secreted immunoglobulin through alternative mRNA splicing. During an antibody response, activated b-cells can switch the expression of CH genes by a type of somatic DNA recombination known as class switching.
2022-04-26T23:06
References
{ Janeway’s Immunobiology, 9th edition Chapter 4.