MHC class I
Subclass of MHC. Specifically binds short peptides of 8-10 amino acids and is present on the surface of all nucleated cells.
MHC Class I molecule expression is increased by interferons
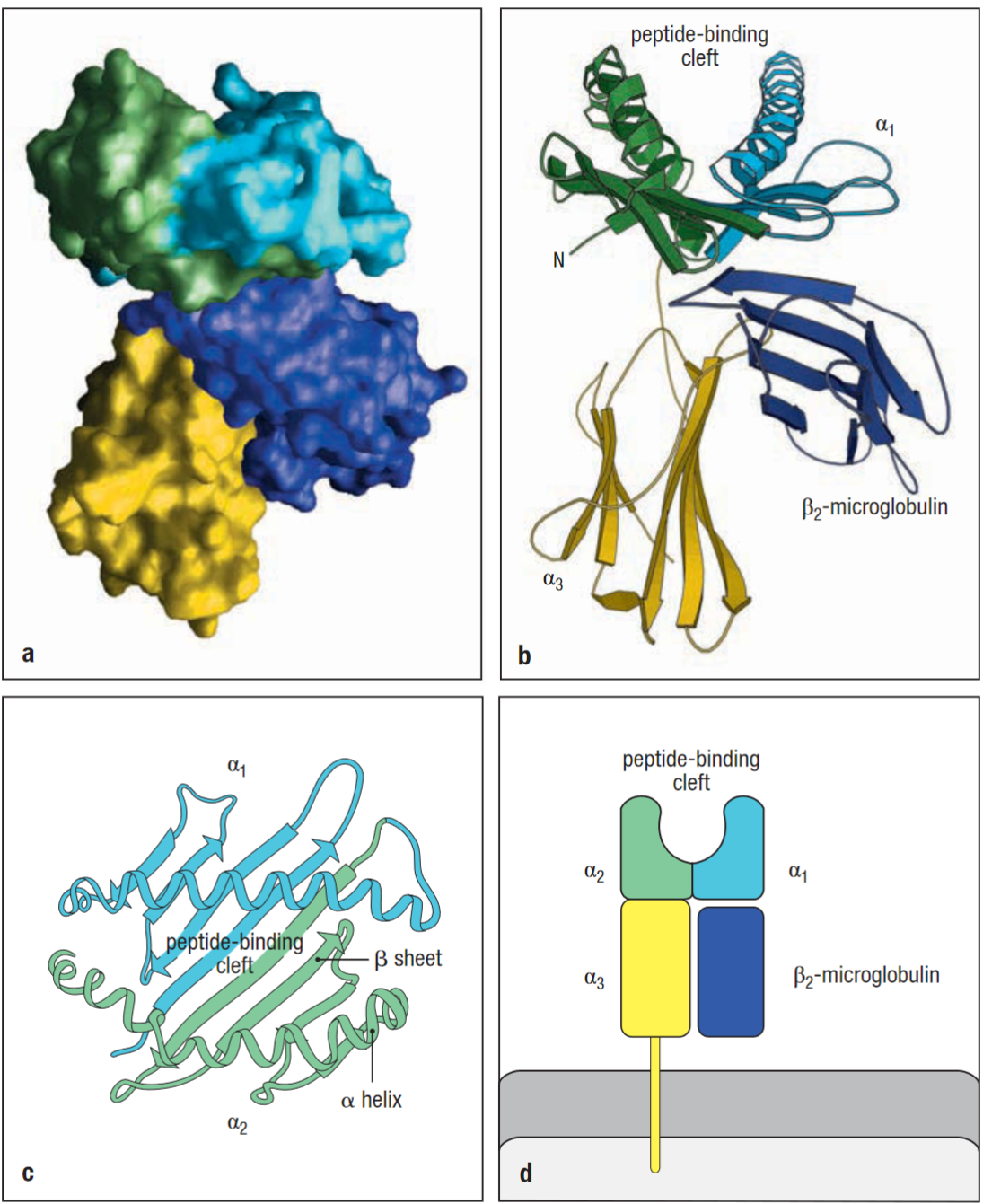
Composed of two polypeptide chains α and β2-microglobulin. Only the α chain spans the membrane in class 1. α3 and β2 resemble Ig-like domains in their structure.
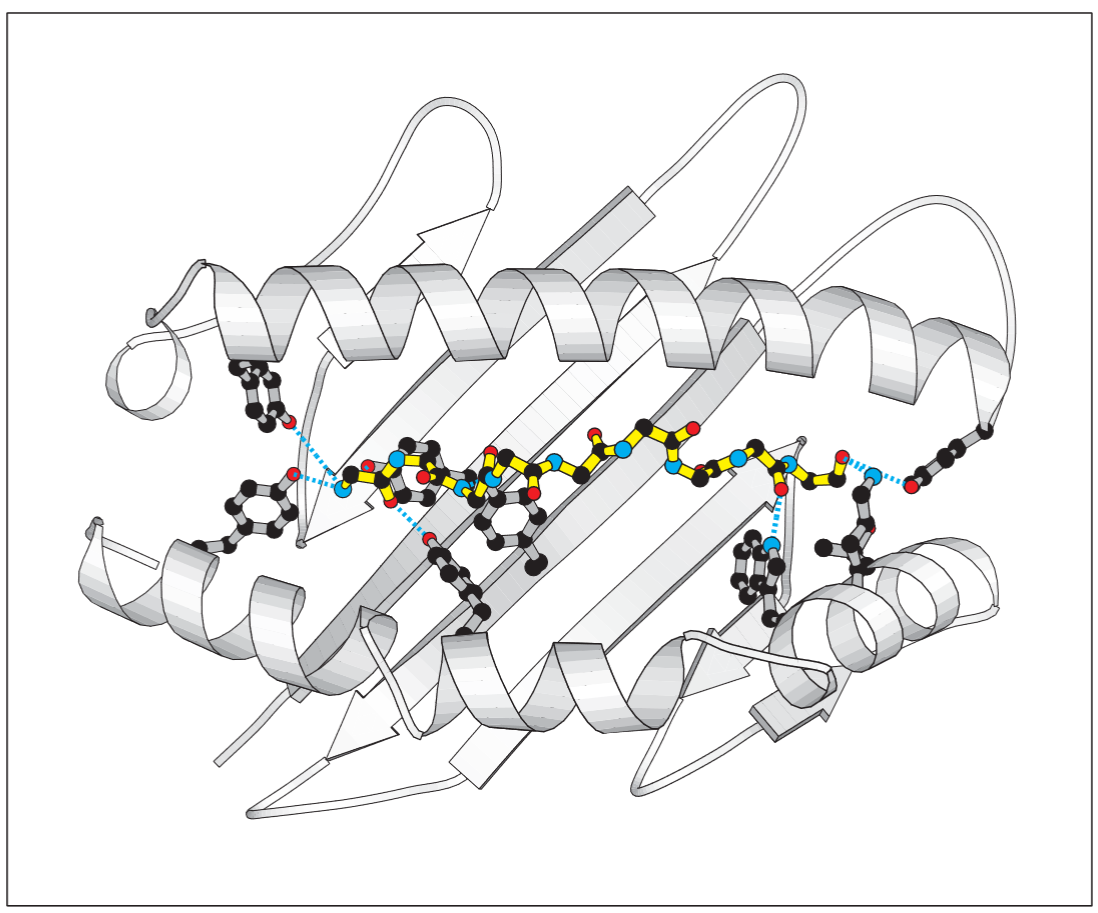
MHC class I molecules interact with the backbone of a bound peptide, through hydrogen bonds and ionic interactions at each end of the peptide. Therefore these invariant ends are thought to be the main stabilising contacts for the pMHC complexes.
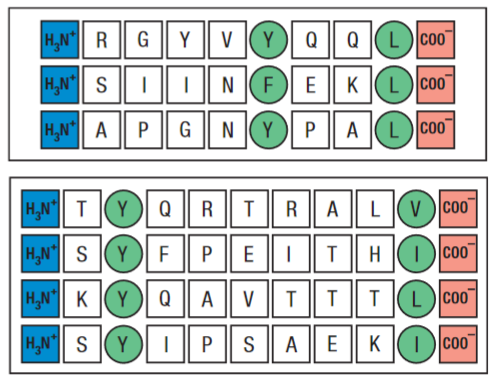
Peptides lacking carboxy and amino terminal groups fail to bind stably. Other residues act as anchors, and these interactions vary depending on the MHC allotype. MHC class I binding peptides are usually 8-10 amino acids long, and if longer peptides bind they are normally cleaved by exopeptidases in the ER.
This use of anchoring gives MHC class I molecules a broad binding specificity. Out of the hundreds of different allelic variations of the MHC class I genes in the human population, only a small selection are carried by each individual.
Info
immunoproteasomes cleave polypeptides after hydrophobic residues to ensure favourable anchor residues for binding to MHC class I.
Peptide:MHC Class I formation
Antigen Processing
Proteases degenerate proteins into short peptide sequences, immunoproteasome can be up-regulated by IFN-γ which can also up-regulate PA28 proteasome-activator complex leading to faster protein degradation.
DRiPs also provide another source of peptides for MHC class I presentation.
The fragments produced during antigen processing are protected from degradation in the cytosol by cellular chaperones such as the TRiC, however many of the peptides produced by the immunoproteasome are longer than can be bound by MHC class I molecules. These longer peptides can be trimmed at the N-Terminus by ERAAP.
Once the peptides have been generated from the native process they are loaded onto MHC molecules in antigen presentation.
Antigen Presentation
Protein chains are translocated during synthesis into the lumen of the ER, where the chains can fold correctly, such as the MHC class I molecule, which is never exposed to the cytosol.
Newly synthesised MHC class I α chains that enter the ER bind to calnexin. Once a β2-microglobulin binds to the α chain, the partly folded heterodimer dissociates from calnexin and binds to an assembly of chaperone protein called the MHC class I peptide-loading complex.
![[CleanShot 2023-12-13 at 07.30.06@2x.png]]
2023-10-13T19:31