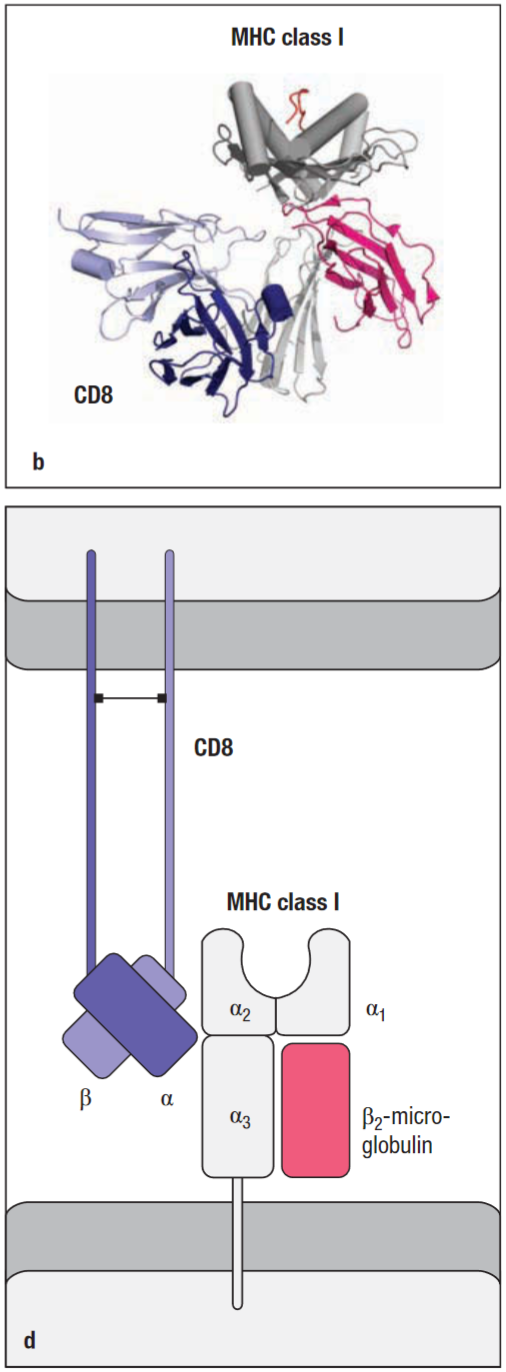
CD8
A disulphide-linked dimer of two chains (α and β), each containing a single Ig-like domain. It is extensively glycosylated to maintain it in an extended conformation and protect it from cleavage by proteases. CD8α chains can form homodimers but these are not normally found when the β chain is expressed.
Tip
CD8αα homodimer can be expressed by activated effector and memory t-cells, as well as mucosal associated invariant t-cells (MAIT cells), which recognise metabolites of folic acid that are produced by bacteria with the nonclassical MHC class I molecule MR1.
CD8 binds weakly to an invariant site in the α3 domain of an MHC class I molecule. The strength of binding of CD8 to MHC is influenced by the glycosylation state of CD8. More sialic acid residues on CD8 carbohydrate structures decreases the strength of the interaction. This likely has a role in modulating antigen recognition.
Similar to CD4, CD8 binds Lck through the cytoplasmic tail of the α chain, enhancing t-cell sensitivity to antigen. (the CD8αα is not effective as a co-receptor and may negatively regulate activation.)